Have you ever wondered why ocean water is salty? What makes our seas and oceans different from freshwater sources like rivers and lakes?
Table Of Contents
−Let’s unravel the mysteries behind the saltiness of our oceans and seas, explore the science, and even uncover fascinating cultural myths about the topic. Buckle up as we embark on a journey through the stories of our seas.

The Natural Water Cycle: The Secret of the Salt
Our story begins with the natural water cycle—a process that has been shaping our planet for millions of years. Here’s how it works:
- Evaporation: Water from the Earth’s surface evaporates into the atmosphere.
- Condensation: Water vapor in the atmosphere condenses to form clouds.
- Precipitation: Water falls back to the Earth’s surface as rain, snow, or other forms of precipitation.
- Runoff: Water flows over land, carrying minerals and salts into rivers, lakes, and eventually the ocean.
This cycle has been repeating itself for eons, shaping our planet’s water systems. But how did the oceans become salty in the first place?
The Two Main Sources of Ocean Salts
- Rock Erosion: Rainwater, slightly acidic due to dissolved carbon dioxide, erodes rocks on land, releasing minerals like sodium, chloride, and calcium. These minerals are carried by rivers into the ocean.
- Undersea Volcanic Activity: Cracks on the ocean floor allow magma to react with seawater, triggering chemical reactions that release magnesium, sulfate, and metallic ions.
Over time, these minerals and ions accumulate, giving the oceans their distinctive saltiness.
Salinity and Its Variables
Salinity refers to the total salt content in seawater, usually expressed in parts per thousand (ppt). For example, a salinity of 35 ppt means there are 35 grams of salt in every 1,000 grams of seawater. But salinity isn’t uniform—various environmental factors influence it:
- Temperature: Warm water promotes evaporation, increasing salinity.
- Rainfall: Heavy rainfall dilutes seawater, reducing salinity.
- Ice Caps: Melting ice caps add freshwater to the ocean, lowering salinity.
- Ocean Currents: Currents distribute salt and heat, affecting salinity levels.
The average ocean salinity is around 35 ppt, but it can vary from region to region. For example, the Baltic Sea, with an abundance of freshwater sources, has a lower salinity of around 10 ppt.
Why Is Saltwater Undrinkable?
While our bodies need both water and salt, drinking seawater is not advisable. The high salt concentration can lead to a condition called hypernatremia, characterized by excessive sodium levels in the blood. Symptoms can include nausea, thirst, muscle spasms, and even death. Seawater also contains potential contaminants that make it unsafe for consumption.
The Salt Crust in the Dead Sea
As a prime example of extreme salinity, the Dead Sea boasts a staggering salinity level of up to 337%! Lacking a connection to freshwater sources and with continuous evaporation, the Dead Sea’s salinity continues to rise, resulting in salt deposits forming on its surface.
Stories and Legends from Ancient Times
Different cultures have their own explanations for the saltiness of the seas. For example, in the Philippines, there’s a folk tale about a salt maker who poured salt into the ocean to punish a greedy sea creature. In Denmark, a legend tells of a magic coffee mill that grinds out salt, causing the ocean to become salty.
Oceans vs. Rivers: The Salt Connection
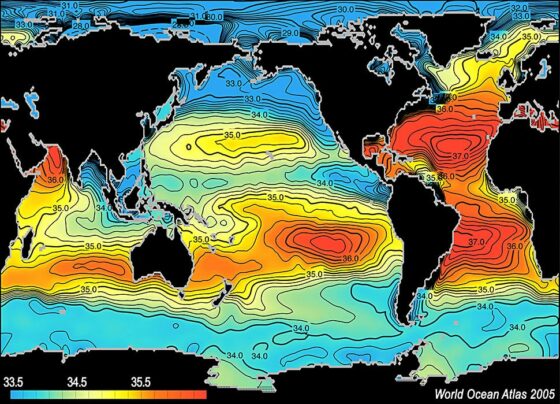
While both rivers and oceans are bodies of water, their salinity levels are strikingly different. Rivers, being supplied with freshwater from rain and melting ice, tend to have lower salinity. As rivers flow, they collect minerals from eroded rocks, but the continuous flow prevents significant salt accumulation.
Oceans, on the other hand, are the ultimate destination for these minerals. Over time, the continuous input of minerals and the lack of an outlet for salts contribute to their higher salinity. As a result, oceans are like vast storehouses for the salts of the Earth.
Practical Examples and Scenarios
Salinity and Marine Life: Salinity plays a crucial role in shaping marine ecosystems. Different species have adapted to varying levels of salinity. For example, corals thrive in saltwater and utilize calcium to build their exoskeletons, while freshwater fish like salmon regulate the salt content in their bodies.
Salinity and Climate: Ocean salinity affects global climate patterns. Areas of high salinity create dense water that sinks and drives deep ocean currents, influencing heat distribution across the planet.
Desalination: As freshwater sources become scarce, desalination—the process of removing salt from seawater to make it drinkable—has gained importance. However, the process requires energy and has environmental impacts, making sustainable desalination a challenge.
Action Items for Further Understanding
- Explore the world of marine life and how different species have adapted to ocean salinity.
- Learn about the technology behind desalination and its potential as a source of freshwater.
- Delve into the cultural myths and legends of different civilizations about the saltiness of the sea.
In Summary
The saltiness of our oceans is the result of a complex interplay between natural processes and environmental factors. Salinity shapes marine ecosystems, influences climate, and challenges our pursuit of drinkable water. From science to culture, the story of the salty seas is one that continues to inspire wonder and curiosity.
We hope this comprehensive article has shed light on the fascinating topic of ocean salinity. Whether you’re pondering the mysteries of the deep or exploring the practical implications for our world, we invite you to dive deeper into the stories of our seas.

Jay
Jay is a health and wellness enthusiast with expertise in water quality and nutrition. As a knowledgeable advocate for holistic well-being, Jay successfully manages Type 2 Diabetes through informed lifestyle choices. Committed to sharing reliable and authoritative insights, Jay combines firsthand experience with a passion for enhancing health."
