Chemistry is a vast world with a multitude of key players, but perhaps no duo is more vital than acids and bases. From the tang of vinegar to the fizz of a soft drink, these substances shape our daily experiences and the world around us. But what makes an acid an acid, and a base? This article unravels the complex world of these foundational chemicals.
Acids and Bases: Unveiling the Basics
Acids: A term derived from the Latin word ‘acidus,’ meaning sour, embodies substances that are sharp to the taste and corrosive to metals. Acids such as vinegar and lemon juice are part of our everyday life, but the acid family extends beyond the pantry. Sulfuric acid powers car batteries, while citric acid lends the tang to fruit juices. Defined scientifically, an acid is any hydrogen-containing material capable of transferring a proton (hydrogen ion) to another substance.
Bases: On the other hand, chemicals that turn blue when added to a mixture of acid and litmus are called bases, or alkalis when they’re soluble. Bases like calcium hydroxide (lime), sodium hydrogen carbonate (baking soda), and magnesium hydroxide (milk of magnesia) are prevalent in household and industrial use. From a chemical standpoint, a base is a molecule or ion that can receive an acid’s hydrogen ion.
Defining the Acid-Base Spectrum
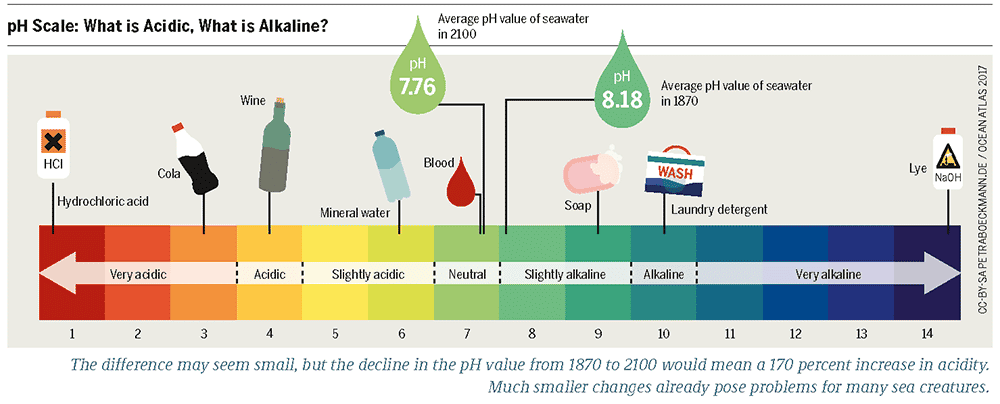
When we talk about acids and bases, we often refer to the pH scale, a measure from 0 to 14 that tells us how acidic or basic a substance is. Anything with a pH between 0 and 7 is considered acidic, while anything between 7 and 14 is regarded as basic. Water, with a pH of 7, is neutral.
Acidic and basic properties are not just abstract concepts; they have practical implications. For instance, acids tend to be corrosive and have a sour flavor, while bases often have a bitter taste and a soapy feel.
The Acid-Base Interaction: A Matter of Hydrogen Ions
At the core of acid and base behaviors is the movement of hydrogen ions (H+). When an acid dissolves in water, it releases hydrogen ions, giving the solution acidic properties. A base, in contrast, can react with these hydrogen ions, reducing the acidic nature of the solution and creating water in the process. This explanation, proposed by Brønsted and Lowry in 1923, continues to inform our understanding of acids and bases.
From Theory to Practice: Acid-Base Interactions in Daily Life
Understanding the chemistry of acids and bases helps us make sense of many phenomena we encounter daily. Let’s look at a few examples:
- The Tang of Vinegar and Citrus: Vinegar and citrus fruits taste sour because they contain acids. Vinegar contains ethanoic acid, while citrus fruits are rich in citric acid.
- The Fizz of Carbonated Drinks: The fizz in your soda comes from carbonic acid, which breaks down into water and carbon dioxide gas when the drink is opened.
- The Soapy Feel of Bases: Bases like sodium hydroxide give the soap its characteristic ‘slippery’ feel. This base reacts with the acids on our skin, neutralizing them and leading to a soapy feel.
- The Corrosiveness of Acids: Some acids can be corrosive. For instance, sulfuric acid, commonly used in car batteries, is a strong acid that can cause burns on contact.
Understanding the intricacies of acids and bases not only enriches our knowledge of chemistry but also deepens our appreciation for the intricate balance of interactions shaping the world around us. Whether it’s the sharp tang of a lemon or the soothing neutrality of water, acids, and bases are central to our everyday experiences. So, next time you bite into a tangy fruit or wash your hands with soap, remember – it’s all just a bit of basic (and acidic) chemistry!

Jay
Jay is a health and wellness enthusiast with expertise in water quality and nutrition. As a knowledgeable advocate for holistic well-being, Jay successfully manages Type 2 Diabetes through informed lifestyle choices. Committed to sharing reliable and authoritative insights, Jay combines firsthand experience with a passion for enhancing health."

